Pharmacovigilance, a vital scientific field, involves detecting, assessing, understanding, and preventing adverse effects in medicines and vaccines. Pharmacovigilance professionals in pharma and hospitals have to read through hundreds of thousands of text passages to search for mentions of adverse events discussed in patient messages, scientific papers, clinical notes, and government registries. The idea here is to detect adverse events and assign them their scientific names and codes and then submit those codes to FDA. The FDA applies statistics to the occurrence of each condition code and calls it a signal if there is a significant causality established. Due to manual reading and adverse event coding, adverse events are heavily underreported which results in delayed post-market surveillance of drug side effects.
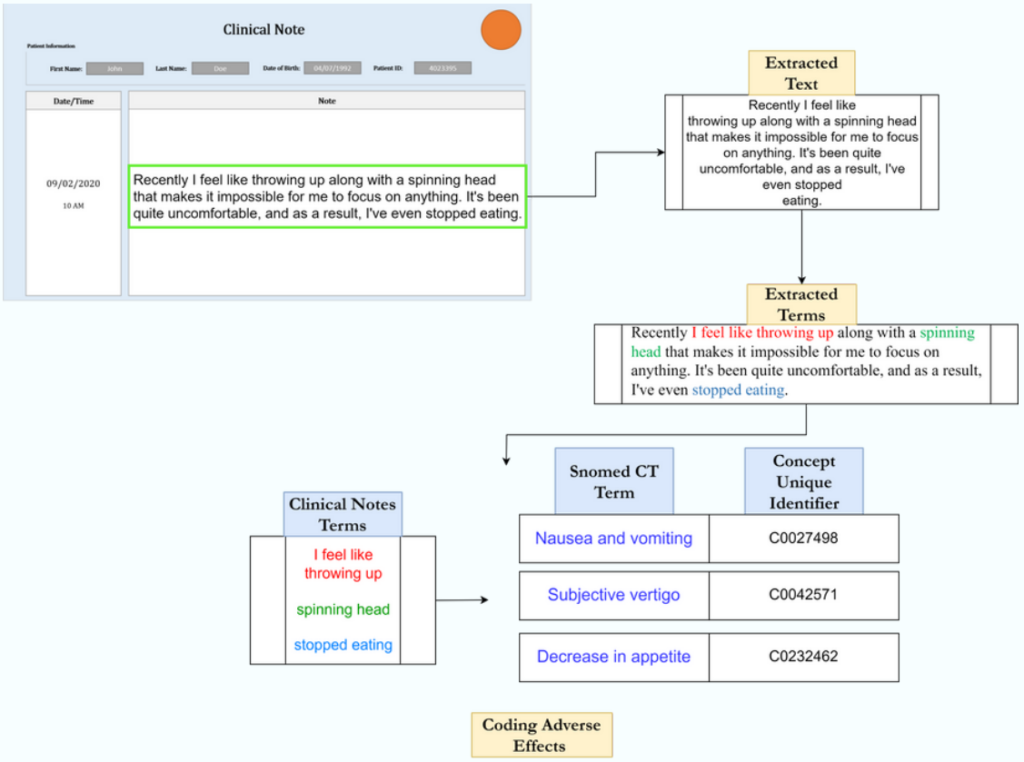
Ingenvio has designed a state-of-the-art solution for adverse events encoding. It automates the pharmacovigilance case report data entry and eventually speeds up the code assignment by 10X.
The FDA is pushing hard to improve the reporting rate of adverse events and is increasingly allowing the use of AI software. The use of AI in the pharmacovigilance process results in:
- Early detection of safety concerns and adverse events.
- Enhanced regulatory compliance and adherence.
- Mitigation of legal and reputational risks.
- Efficient identification of potential drug interactions.
- Minimization of product recalls and associated costs.